A Sample of Zinc Metal Reacts Completely With
The amount of. A sample of zinc metal reacts completly with an excess of hydrochloric acid.

Chemical Reactions Ppt Download
Z n s 2 H C l a q Z n C l 2 a q H 2 g beginalign mathrmZns2mathrmHClaqrightarrowmathrmZnCl_2aqmathrmH_2g endalign Zn s 2 HCl a q ZnC l 2 a q H 2 g.

. The reaction takes place according to Zn 2 HCl ZnCl2 H2. Zns 2HClaq -- ZnCl2aq H2g The hydrogen gas produced is collected over water at 250 C. 1 mol of Zn has mass of 6539 g.
Calculate the amount of zinc metal in grams consumed in the. A sample of zinc metal reacts completely with an excess of hydrochloric acid. Assume the specific heat of the solution is 4184.
Binary compounds of alkali metals and hydrogen react with water to liberate. A sample of zinc metal is allowed to react completely with an excess of hydrochloric acid. Calculate the amount of zinc metal in grams consumed in the reaction.
Add To Playlist. Z n s 2 H C l a q Z n C l 2 a q H 2 g The hydrogen gas produced is collected over water at 250 C using an arrangement similar to that shown in Figure 1114 a The volume of the gas is 780 L and the pressure is 0980 atm. Zinc reacts with sulphur to form zinc sulphuric 162gSample of zinc is reacted with log of sulphurZn65S32o16 AWrite a chemical reaction for the reaction bCalculate the number of moles of zinc atoms that reacted.
The acid quantity of 55 g can react with 654 55 73 493 g of Zn and 057 g Zn remain unreacted. So for equal masses of Zn and HCl the metal is în excess. When zinc is added to copper sulphate CuSO 4 solution due to more reactivity of zinc copper is replaced by zinc and forms zinc sulphate.
A sample of zinc metal reacts completely with an excess Zns 2HClaq ZnCl2aq H2g The hydrogen gas produced is collected over water at 250C using an arrangement similar to that shown in Figure 515. Copper Zinc Sulphate No reaction dSodium and potassium are stored in kerosene. General Chemistry 6th Edition Edit edition Solutions for Chapter 5 Problem 64Q.
MathrmZns2 mathrmHCla q longrightarrow mathrmZnCl_2a qmathrmH_2g. A 0158 g sample of magnesium metal reacts completely with 1000 mL of 10 M hydrochloric acid in a coffee cup calorimeter. Zinc reacts with hydrochloric acid according to the reaction equation.
Write the balanced chemical equationb. Since zinc is more reactive than copper copper cannot displace zinc from its salt solution. MathrmZns2 mathrmHCla q longrightarrow mathrmZnCl_2a qmathrmH_2g The hydrogen gas produced is collected over water at 250circ mathrmC using an arrangement similar to that shown in Figure 1014mathrma.
SOLVEDA sample of zinc metal reacts completely with an excess of hydrochloric acid. Zns 2HClaq -- ZnCl2aq H2g The hydrogen gas produced is collected over water at 250 C. CNumber of moles of sulphur atoms.
The volume of the gas is 780 L and the pressure is 0980 atm. Zns 2HCIaq ZnCl2aq H2g The hydrogen gas produced is collected over water at 25C using an arrangement similar to that shown in Figure 515. A sample of zinc metal reacts completly with an excess of hydrochloric acid.
780 L of the gas was collected at a total pressure of 0980 atm. Zinc has an atomic mass of 654 g and the sum of two molecular masses of HCl makes 73 g. During the process the colour of the solution changes from blue to colourless.
The volume of the gas is 780 L and the pressure is 0980 atm. And the atmospheric pressure is 0980 atm. Calculate the amount of zinc metal in grams consumed in the reaction.
Up to 256 cash back 5. In the following chemical reaction zinc oxide reacts with carbon to produce zinc metal and carbon monoxideZnO CZn COa Name the substance getting oxidised and reduced in the above reactioni C and ZnOii Zn and Ciii ZnO and COiv CO and ZnOAnsZnO CZn CONowAddition of oxygen i. The hydrogen gas produced is collected over water at 250C.
A sample of zinc metal reacts completely with an excess of hydrochloric acid 0247. A sample of zinc metal reacts completely with an excess of hydrochloric acid. A sample of zinc metal reacts completely with an excess of hydrochloric ZnCl2aq H2g The hydrogen gas produced is collected over water at 250C using an arrangement similar to that shown in Figure 515.
Zn 2HClaq ZnCl H2O The hydrogen gas was collected over water at 25C. A sample of zinc metal reacts completely with an excess of hydrochloric acid. Calculate the amount of zinc metal in grams.
A sample of zinc metal reacts completely with an excess of hydrochloric acid. A sample of zinc metal reacts completely with an excess of hydrochloric acid. The volume of the gas is 780 L the pressure is 0980 atm.
If sodium peroxide is added to water elemental oxygen gas is generated. What volume of hydrogen at STP is produced when 25 g of zinc react with an excess of hydrochloric acid in the reaction Zn 2HCl - ZnCl_2 H_2. C Copper cannot displace zinc from its salt solution.
19 A sample of Zinc metal reacts completely with an excess of Hydrochloric Acid as shown in the reaction below. Zns2HClaq-ZnCl2aqH2g How many milliliters of 450M HCl1aq are required to react with 825g of an ore containing 300 Zns by mass. Mathrm Zn s2 mathrm HCl a q longrightarrow mathrm ZnCl_ 2 a qmathrm H_ 2 g The hydrogen gas produced is collected over water at 250 circ mathrm C using an arrangement similar to that shown in Figure 1114 mathrm a The volume of the gas is 780 mathrm L.
Note that the vapor pressure of water at 200C is 17535 mmHg. Zn s 2HCl aq --- ZnCl2 aq H2 g The hydrogen gas produced is collected over water at 250 degrees C. A sample of zinc metal is allowed to react completely with an excess of hydrochloric acidThe hydrogen gas produced is collected over water at 250C using an arrangement similar to that shown in Figure 514.
1 Answer Bio Feb 28 2016 086 L Explanation. The volume of the gas is 780 L the pressure is 0980 atm. The temperature of the solution rose from 256C to 328C.
The vapor pressure of water at 25C is 238 mm Hga. The volume of the gas is 780 L. The wet pressure of the collected gas was 745 mm of Hg and the volume was 780 Liters.
A sample of zinc metal reacts completely with an excess of hydrochloric acid. Chemistry Gases Molar Volume of a Gas. A sample of zinc metals reacts completely with excess hydrochloric acidZns 2 HClaq ZnCl2aq H2g If 7802 mL of gas is collected over water at 200C and a pressure of 7540mmHg determine the initial mass of zinc used in grams.
The chemical equation of the reaction. In an aqueous solution a high reactive metal can displace a less reactive metal from its salt.

Reaction Of Zinc With Hydrochloric Acid Chemistry Demonstration Youtube

Solved Zinc Metal Reacts With Hydrochloric Acid To Produce Chegg Com

Metals Reacting With Acid Video Khan Academy

10 Zinc Metal Reacts With Hydrochloric Acid To Form Zinc Chloride And Hydrogen Gas A Write Homeworklib
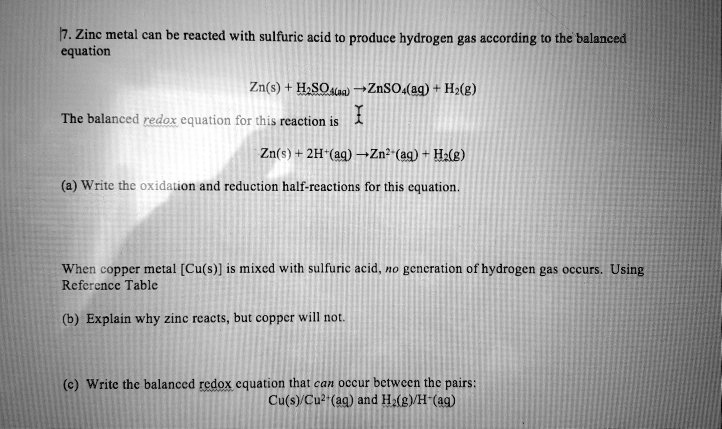
Solved Zinc Metal Can Be Reacted With Sulfuric Acid To Produce Hydrogen Gas According T0 The Balanced Equation Zn S Hsqav Znsoa Eq Hx G The Balanced Redox Equation For This Reaction Is Zn S 2h Aq

Please Help 2 When Zinc Metal Reacts With Copper Ii Nitrate Zinc Ii Nitrate And Copper Metal Are Homeworklib

Solved Zinc Metal Reacts With Hydrochloric According To The Balanced Equation Quad Mathrm Zn S 2 Operatorname Hcl A Q Longrightarrow Mathrm Zncl

10 Zinc Metal Reacts With Hydrochloric Acid To Form Zinc Chloride And Hydrogen Gas A Write Homeworklib
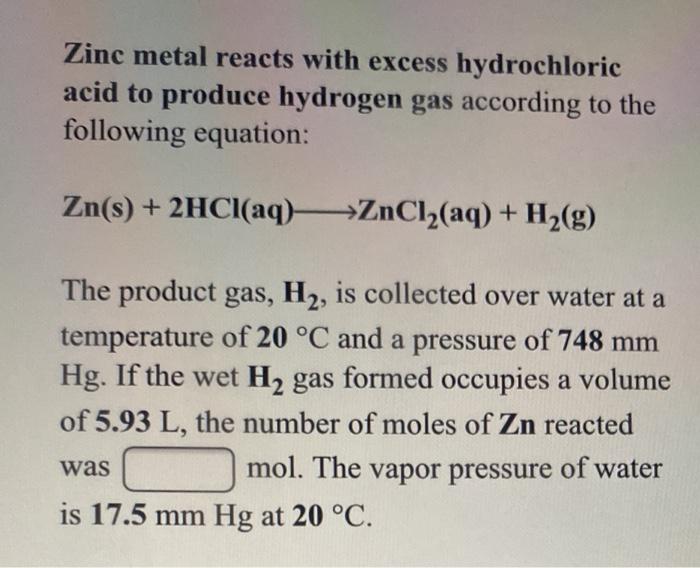
Solved Zinc Metal Reacts With Excess Hydrochloric Acid To Chegg Com

Solved B A Sample Of Zinc Metal Reacts Completely With An Chegg Com

Pin By Sanghita Dey On Cbse Class 10 In 2021 Solutions Homogeneous Mixture Bullet Journal
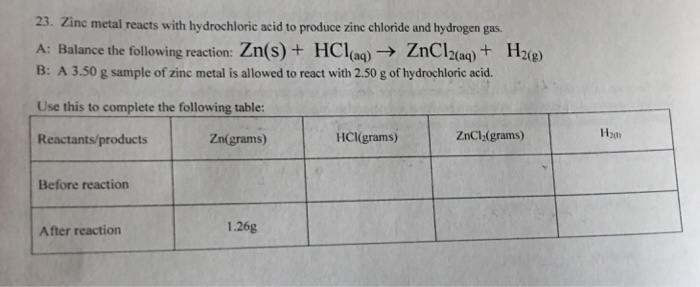
Solved Zinc Metal Reacts With Hydrochloric Acid To Produce Chegg Com
Zn Hcl Reaction Zinc Hydrochloric Acid Youtube

Answered 2 Aluminum Metal Reacts With Zinc Bartleby

Pin On Amazing Facts Of Science

Solved Consider This Reaction Zinc Metal Reacts With Chegg Com

Solved A Sample Of Zinc Metal Reacts Completely With An Excess Of Hydrochloric Acid Mathrm Zn S 2 Mathrm Hcl A Q Longrightarrow Mathrm Zncl 2 A Q Mathrm H 2 G The Hydrogen Gas Produced Is Collected Over Water At 25 0 Circ Mathrm C
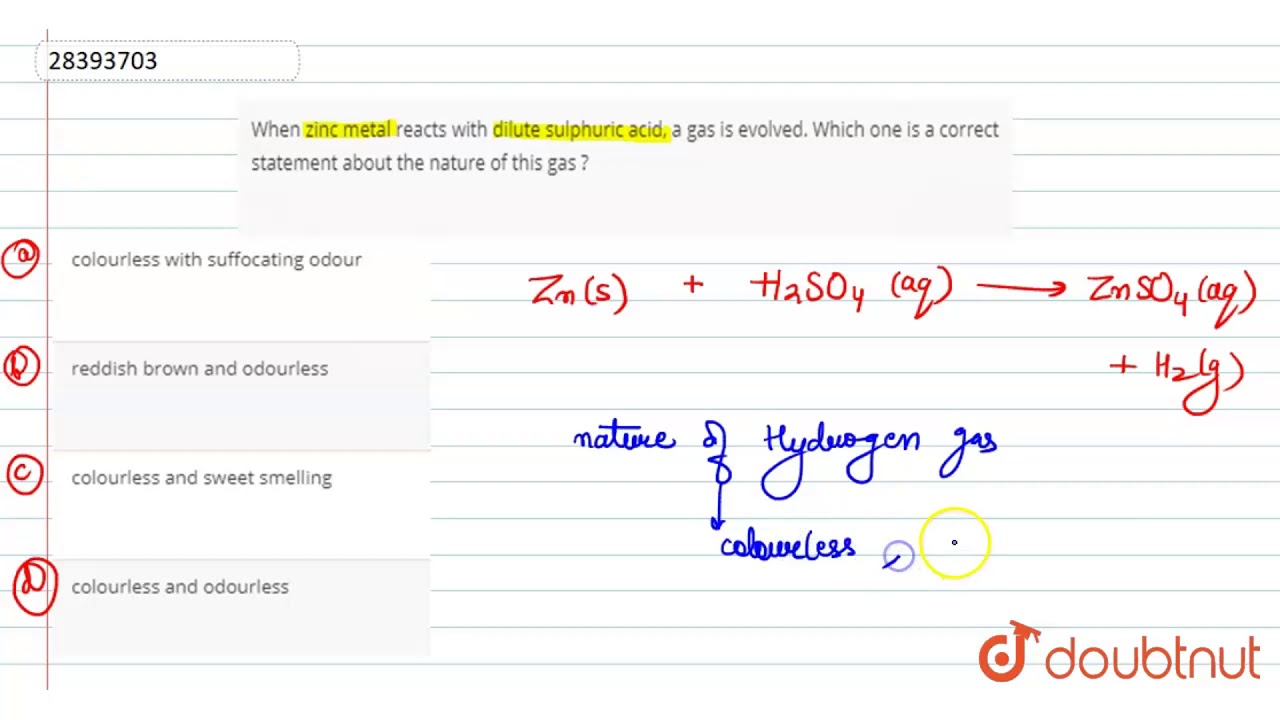
When Zinc Metal Reacts With Dilute Sulphuric Acid A Gas Is Evolved Which One Is A Correct Youtube

Comments
Post a Comment